Our Aims
- To address the current scarcity of research on congenital anomalies, particularly from low- and middle-income countries (LMICs), by undertaking the first large series, geographically comprehensive multi-centre prospective cohort study of congenital anomalies across the globe. Such data is vital to inform advocacy efforts and global health prioritisation.
- To identify factors affecting outcomes of children with congenital anomalies to improve care.
- To enhance research capacity amongst collaborators and combat the huge disparity in research outputs between low-, middle- and high-income countries at present.
- To form a global paediatric surgical research collaboration to enable further research and intervention studies aimed at improving outcomes.
Why Congenital Anomalies?
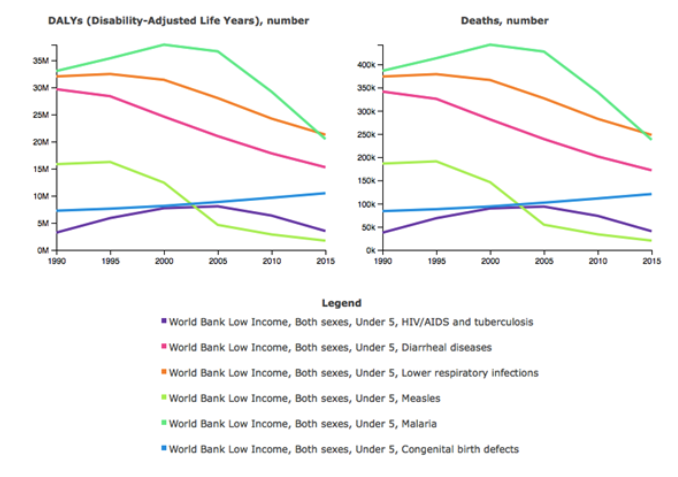
In 2015, the Global Burden of Disease Study reported that congenital anomalies have risen to become the 5th leading cause of death in under 5-year olds globally. In real terms, this equates to almost half a million deaths/ year, with 97% of those deaths in LMICs.
At present, there is a stark scarcity of research on congenital anomalies from LMICs. Taking congenital diaphragmatic hernia as an example, we could identify just 14 studies on this condition from LMICs. Yet hundreds of studies have been published on the same condition from high-income countries. In addition, the LMIC studies that have been published are mostly single institution, small series, retrospective studies.
Joining the Study
If you are interested in participating, please contact Dr Naomi Wright (Principal Investigator) at paedsurg.research@gmail.com for further information and to sign up.
For further updates, please follow us on Twitter or Facebook.
Collaborators are invited to participate from a wide range of organisations. Any healthcare professional who is involved in the care of infants presenting primarily with congenital anomalies can participate in the study i.e. surgeons, anaesthetists, neonatologists, paediatricians, trainees, medical students and nurses.
There can be up to 3 collaborators per institution per month of data collection. Hence, there can be more than one team of 3 collaborators collecting data over different time points between Oct 2018 – April 2019.
All collaborators will be included as PubMed citable co-authors. The study will also be used to create opportunities for collaborators. For example, collaborators may request to present the study (initially the concept, and later the results) at national, regional and international conferences. This often provides collaborators with the opportunity to apply for funding through the conference organisation to attend and present. Following the study, online training will be provided to collaborators on how to set up their own projects using REDCap. For collaborators who are interested, an optional research training fellowship will be offered alongside the main study including monthly online webinars and one-on-one mentorship with an academic.
Collaborator Roles
There are many ways in which to get involved in this forthcoming study:
1) As a Local Collaborator
This involves using the study protocol (to be circulated in the coming days) to apply for and gain local approval to participate in the study. There can be up to 3 collaborators per centre per month of data collection. Data collection will be on all patients presenting primarily with one of the seven study conditions over a minimum of 30-consecutive days of collaborators choice between Oct 2018 and April 2019.
Data collection forms will be provided and data is to be entered into the user-friendly, free of charge, secure online database REDCap. You will be provided with login details once study approval has been secured at your institution. There will be approximately 30 data points per patient to minimise the time required to participate in the study. At 10% of centres the team will also be asked to identify an independent collaborator to collect a selection of data again into a validation database, which will be used to determine the accuracy of the data collected. This is required for publication.
Collaborators will have the opportunity to present the study at meetings and conferences across the globe – initially the study concept to recruit collaborators and then subsequently the study results once it is complete. This will be co-ordinated by the organising committee to avoid duplications and to ensure all standards and regulations are complied with.
2) As a Country Lead
In addition to the roles of a local collaborator, a country lead helps to recruit other collaborators to participate in the study from across their country. They also help to trouble-shoot with questions from local collaborators and may help to provide support with gaining local study approval. Another role may be to help translate the study literature into the local language of the country if required.
3) As a Regional or Continent Lead
In addition to the roles of a local collaborator, a continent lead will help to recruit country leads. They will act as a first port of call for country leads who have questions regarding the study. They will encourage and co-ordinate presentations of the study protocol at national and international meetings and conferences within their continent to help recruit collaborators. Following such presentations, they will help to direct interested collaborators to the appropriate country lead for further advice.
Any questions or queries can be forwarded onto the Principal Investigator (Naomi Wright) and the organising committee at paedsurg.research@gmail.com.
4) As a Lead Organiser
- Developing the study logo.
- Developing and maintaining a ‘Global PaedSurg’ website.
- Co-ordinating a blog on the website with contributions from collaborators from around the world.
- Translation of study documents, the REDCap data collection tool and the website to optimise inclusivity into the study of all countries around the world.
- Development of the REDCap data collection tool and translation into different languages.
- Running the Global PaedSurg Twitter account.
- Initiating and running a Global PaedSurg Facebook account.
- As above for other social media outlets.
- There will be an opportunity for a group of collaborators to write up the study protocol for publication in advance of data collection.
- Maintaining the database of collaborator details and communicating with collaborators.
- Co-ordinating the national, regional and international meetings.
- Opportunity to present at conferences globally to recruit collaborators.
- Helping with co-ordination and documentation for the research training fellowship.
5) As a Lead Investigator
In addition to the roles of a local collaborator:
- Participation in the pilot study and provision of feedback on how to optimise the data collection forms and study prior to study launch in October 2018.
- Participation in the pilot study and design of the data validation process.
- There will be an opportunity to help write up the study protocol for publication in advance of data collection.
- Contribution to the writing and revisions of the results manuscript for publication.
